Dr. Shay Laps* and Prof. Danny H-C Chou*
Department of Pediatrics, Division of Diabetes & Endocrinology, School of Medicine, Stanford University, Palo Alto 94304, USA.
*Corresponding authors: shaylaps@stanford.edu ; danychou@stanford.edu
Graphical Abstract
Abstract
Insulin – a hormone, a medication, and protein – is one of the most fascinating and influential molecules ever discovered. Scientific understanding of insulin and the ability to adapt it for medical needs cross boundaries of knowledge, technology, and research. For hundreds of millions of people living with diabetes around the world, it is not only a scientific achievement but an urgent necessity – a vital solution that saves lives every single day. The chemical functionalization of insulin has been desired for various applications such as controlled release (e.g. short or long acting), rescue from toxic fibrillation, and searching for alternative administration approaches (e.g. oral medication). In Type-1 diabetes the controlled pancreas mediated secretion of insulin is been diminished, raising the need to apply insulin to the blood circulation on demand and careful control. With the risk of hypoglycemia attempts are being explored continuously to develop chemistry for the rewiring of insulin properties towards gaining an unnatural sensitivity to glucose blood levels, mimicking the failed pancreatic control. Due to their sugars binding capabilities, boronic acids are great candidates for potential insulin built-in glucose molecular sensors. Nevertheless, despite a century of scientific research the synthetic information currently available for multiple and selective insulin modification is inadequate, limiting the possibility to explore the effect of sensitive functional groups implantation into insulin. Moreover, boronic acid compounds were discovered to be less potent towards glucose rather than other sugars (e.g. fructose), limiting its therapeutic potential. Here, we report the development of a de novo Protein Specific Modification (PSM) strategy. The strategy focuses on shifting the paradigm from general-to- specific protein modification protocols. We revealed and utilized a new synthetic kit to perform multiple insulin modification in a precise manner. Utilizing our developed protocol, we were able to modify naked insulin precisely with three different elements. Moreover, we have progressed towards the potential development of smart boronate insulin by the introduction of synthetic rigid diborontae motif with glucose sensitive properties. Also, we have inserted a glucose mimic element to explore potential activity changes upon internal reversible bridging in presence and absence of glucose. Our in-cell activities findings reveal surprised tolerance of two de novo insulin analogues to a fluctuation with sugars levels. We anticipate these findings to pave the way for the further exploration of synthetic diboronate chemistry in the context of insulin modification. In addition, we expect our PSM approach for targeting a solely protein or a member of large protein family to transform the field of protein chemistry, allowing precise tools for more focused aims, with tremendous therapeutic potential and scope. This paradigm shifts from the general to the specific targeted chemistry in peptide and protein science is anticipated to open the door for a future database, we term as; PSM bank, to modify specific protein in the presence of other peptide and proteins, potentially for in vivo spatial selectivity.
Introduction
Since its discovery more than a century ago, insulin has transformed our world in economic, societal and scientific perspectives.1 Its properties and behavior in the molecular level driven numerous studies to reveal its uniqueness among the proteome. Such as the importance of the mission to mars or the development of ships to cross oceans, the aim to understand and control insulin for the benefit of humanity, challenges scientists globally.1–3 For example, most people with type 1 diabetes (T1D) necessitate exogenous insulin injections to manage their glycemic levels.4 Despite extensive and rigorous research over an extended period, diabetes management remains a significant challenge as it hinges on a combination of insulin-based treatments and patient’s self-regulation.4 Current insulin therapies strive to emulate pancreatic insulin secretion by employing distinct fast-acting and long-lasting insulins to preserve glycemic homeostasis during meals, intermeal periods, and across day-night cycles. Achieving tight glycemic control demands that fast-acting insulins react swiftly to increased blood glucose concentrations, thereby avoiding hyperglycemia.5 Simultaneously, long-lasting insulins must supply consistent basal activity within a therapeutic window of 24 h to facilitate once-daily dosing. However, a major limitation of the existing insulin analogs is the lack of glucose-dependent activity regulation. Consequently, individuals with T1D are forced to make imprecise “estimations” of their insulin requirements, which often results in either excess or insufficient medication.6 Excess insulin administration can result in hypoglycemia due to inability of current available insulin analogs to modulate their activity even under low glucose levels. Hypoglycemia poses a substantial risk as it can precipitate acute complications such as unconsciousness, coma, and potentially, death. It is estimated that hypoglycemia is responsible for the death of 2-10% of individuals with T1D.5 Fear of this severe complication often leads to under-dosing of insulin and, consequently, suboptimal management of diabetes. The resultant persistent hyperglycemia fosters complications like blindness, renal failure, and cardiovascular disease. Therefore, an innovative therapeutic approach that robustly and dynamically modulates insulin activity to more closely align with fluctuating blood glucose levels is urgently required.
While the selective modification of insulin to control and modulate its biological performance requires the aid sophisticated synthetic tools,7–10 the aim of creating glucose responsive insulin (GRI), also termed; smart insulin, sparked the imagination for decades.11–16 GRI would have the ability to self-sense glucose blood levels and switch from an active to inactive states to maintain normoglycemia, while protecting patients from hypoglycemia. Despite the progress that has been made towards this aim, the insensitivity nor specificity towards glucose rather than other sugars (e.g. fructose), the complicated and tedious synthetic roads to obtain such leads and the nature of the composites hindering their potential to been utilized in humans as the future diabetes therapy.11,14
Given the considerable potential implications of GRI in diabetes treatment, several approaches have been initiated to tackle this challenge. Predominantly, GRI systems under investigation leverage polymeric biomaterials that incorporate insulin into a matrix featuring glucose-responsive elements such as glucose-binding proteins (GBP), glucose oxidase (GOx), and Phenylboronic acid (PBA).17–19 These elements can modulate the rate of insulin release by altering the matrix structure, initiating polymer degradation or glucose binding competition. Also, the emergence of DNA nanotechnology state-of-the-art approaches paved the way for utilizing these features for the controlled release of insulin from hydrogels and microcapsules.20,21 In particular PBA has shown remarkable qualification for administration into humans and binding sugars reversibly.18,22–24 This chemical feature has been utilized previously to develop proof of concept responsive insulin materials, however no glucose selectivity has been ever reported.12,18 We envisioned the design of an insulin motor, switching from an active to inactive form by introducing precisely into human native insulin skeleton glucose binding mimic element and a glucose sensitive sensing element. Rigid diboronate motif has shown high binding selectivity towards glucose in the presence of other sugars (e.g. fructose). In particular, [1-[2-[[2,5-bis[(3-boronopyridinio)methyl]benzoyl]oxy]ethyl]-2,5- dioxo-3 pyrroleidione (DiPyBA) has been recently proven to sense glucose efficiently in small molecule studies.25,26 Inspired by previous experiences of our lab and others, we chose 3,4- dihydroxybenzylic motif bearing 1,2 cis-diol to serve as potential effective internal mimetic for glucose.27 On the other hand, we hypothesized that selective implementing of DiPyBA into insulin will allow a structural fluctuation based on a reversible interaction between DiPyBA and the catechol in presence and absence of glucose under chemoselective recognition. Nevertheless, the synthetic challenges associated with the design of an insulin bearing these features due to the instability and sensitivity of the mentioned above elements and the complication of insulin handling regardless (e.g. prone for aggregation) required us the exploration of effective chemical kits.
Rather than peptides and proteins in general, more specific problems are being encountered upon considering strategies to modify insulin multiple times in a site selective manner. The toolbox for the general site-specific protein modification has continually been growing, while enabling controlled chemistry to distinguish between the various reactive functional groups with a protein sequence.7,16,28–36 For example, a precise selection between N- terminal and the ε-amino side chain of lysine (Lys) has been demonstrated upon the natural pKa differences between the N-terminal amine (~8) in contrast to Lys (~10). Selective cysteine (Cys) or selenoCys (Sec) residues modification strategies have been extensively reported.29,37–
41 Despite these, the available general synthetic repertoire simply cannot be used in specific proteins in various cases, due to less general properties and variation. This is what we have encountered with insulin due to three main factors: (1) The presence of two N-terminal amines in one protein, (2) its poor solubility properties and tends for complexation (e.g. dimerization) and aggregation prone, (3) a fluctuation on pKa values, dependent upon various reaction conditions (e.g. concentration or temperature).42
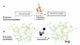
Figure 1. Previous approaches for the multiplex modification of insulin, utilizing a combination of enzymatic and chemical tools, versus the current work, utilizing fully synthetic protocol.
Intrigued by these, we have focused our attention on exploring synthetic schemes to fit perfectly (and perhaps only) to insulin. Thus, we have developed Protein Specific Modification (PSM) protocol for insulin. It should be noted that targeting a solely protein or a member of large protein family and its exploration for PSM development, could transform the field of protein chemistry, allowing precise tools for more focused aims, with tremendous therapeutic potential and scope. For example, the conotoxin families, defensin antimicrobials and ubiquitin likes. This paradigm shifts from the general to the specific targeted chemistry is anticipated to pave the way for a future database, we term as; PSM bank to modify specific protein in the presence of other peptide and proteins, potentially for also in vivo spatial selectivity.
Results and Discussion
Previous reports about selective attachments of multiple elements into insulin have been utilized mainly enzymatic recognition of targets tags.43 Despite its importance and utilities in various other than insulin examples, these enzymatic transformations are not allowing the insertion of small molecules without applying wisely protection/deprotection schemes. Thus, complicating the synthetic protocol. It should be noted that the operation of enzymatic based processes is limited to folded and active enzyme environments and therefore potentially limiting the substrate scope. From a societal point of view, the increasingly growing incidents of diabetes and the demand for insulin raises the unmet need for simpler, efficient chemical- based processes to modify insulin, which could be potentially operated on industrial scales.10 Despite the progress in the challenging synthesis of small Cys rich miniproteins and the potential to allow future insulin total synthesis in more effective ways,8,10,30,38,39,44–49 up to date the most effective way for insulin production remains utilizing recombinant strategy.1,24 Therefore, we focused our attempts to investigate opportunities for the multiple modification of a recombinant human insulin.
Targeting precisely insulin B1 N-terminal amine
We have envisioned, based on structural and computational information,14 that a modification on insulin with its N-terminal amine located on the heavier chain (B chain) would only slightly interfere with its potency in contrast to the A chain case. Experimentally, our lab discovered that the truncation of this chain up to His5 retains its anticipated biological signaling in situ (data not shown). Therefore, we attempt to specifically introduce modifications at this site. 3,4 dihydroxy benzaldehyde (DHBA) has been chosen, having both potential for mimicking glucose 1,2-cis diol frame and slightly reducing global hydrophobicity. The common knowledge for pKa values of N-terminal amine and the ε-amino within Lys side chain, enabled various general studies. However, in the case of insulin, the pKa values turned out to be fluidic. The environment including temperature and protein concentration influence the pKa’s values.42 As part, such interesting phenomena can contribute to insulin-insulin interaction while changing the effective surrounding of these amines. Based on these, we have initiated reductive amination chemistry applying DHBA to specifically modify B1 N-terminal amine. The reaction was performed at 0.1 M citric acid buffer, pH 5, 25oC. recombinant human insulin was dissolved initially in 6 M Guanidine HCl, 200 mM phosphate buffer (Gn.HCl), pH 7.5 and further diluted into 1 mM final protein concentration. 5 equiv. DHBA in the presence of 10 equiv. NaCNBH3 provided single modified insulin after 27 h (Figure 1).

Figure2.LCMS traces for site-specific insulin B1 N-terminal modification.
Exploring site selective lysine modification
Next, we have aimed to perform a second modification precisely. Our design included the utilization of the glucose-boronic acid reversible interaction. Despite notable examples of boronic acid as a lead compound towards smart insulin creation, mostly fructose rather than glucose stimuli responsiveness has been reported. We hypothesized the incorporation of DiPyBA directly to insulin to allow for the first time real boronic acid based GRI. Here, we have synthesized DiPyBA bearing two boronic acids in rigid assembly, found previously to sense glucose. Notably, DiPyBA hydrophilic properties potentially induce new structural information, rewiring insulin natural prone for fibrillation. Such a molecule potentially will rewire insulin to sense glucose, towards glucose responsive insulin therapy. The synthesis of DiPyBA performed following previous protocol26 with an additional optimization (Figure 3, SI). To examine its compatibility with peptide modification and purification we have synthesized a model study case as follow: Fmoc- solid phase peptide synthesis (SPPS)30 has been employed to produce the peptide GGG(Dap(ivDde))[PEG9]CGGSLEEEWAQIQSEVWGRGSPSY, derived from a non- insulin-peptide with insulin receptor activation features.50,51 3,4-dihydroxyphenylacetic acid (DOPAC) was conjugated to the peptide on solid support (SI), starting with a protected peptide bearing 2,3-diaminopropionic acid (Dap)(ivDde) followed by ivDde deprotection and DOPAC coupling. The resin containing Dap(ivDde) peptide (0.1 mmol) was suspended and rotated in a solution of 5% hydrazine in DMF (4.0 mL) at room temperature for 0.5 h. After washed with DMF (5 mL) 3 times, the resin was suspended and rotated in a solution of DOPAC (46.24 mg, 0.3 mmol), DIC (46.97 μL, 0.3 mmol), and HOBt (40.54 mg, 0.3 mmol) in DMF (4.0 mL) at room temperature for 18 h followed by washing with DMF (5 mL) 3 times to yield a peptide containing Dap(DOPAC) on resin.30,52 Next a mixture of cleaved peptide GGGX[PEG9]CGGSLEEEWAQIQSEVWGRGSPSY (1.9 mg, 0.54 μmol) and 4(0.73 mg,
1.08 μmol) in water (0.54 mL) was stirred at room temperature for 3 h, and then purified by HPLC on a C18 column with a 10-50% linear gradient of a solution of 0.1% TFA in CH3CN/H2O to yield a white solid product GGGX[PEG9]ZGGSLEEEWAQIQSEVWGRGSPSY. (1.1 mg, 0.27 μmol, 50%). ESI-MS calc. monoisotopic mass 4030.77, obs. [M-4H2O+3H]3+ 1320.3, [M-4H2O+4H]4+ 990.5. X stands for Dap(DOPAC). Zstands for Cys(Su-DiPyBA).

Figure 3. HNMR analysis for synthetic DiPyBA and its incorporation into a synthetic model peptide: GGGX[PEG9]ZGGSLEEEWAQIQSEVWGRGSPSY.
To incorporate DiPyBA into insulin a thio-Michael addition chemistry should be applied, however no free Cys is available within insulin sequence. A reduction of disulfide bonds would result in structure and activity lost. Thus, an insertion of Cys should be devised. We have chosen Lys at B29, due to previous studies showing variation at this site should accommodate with insulin natural signaling. While generally the pKa for such site would be considered relatively high (~10), the associated harsh basic conditions are incompatible with sensitive molecules to be attached (e.g. activated carboxylate, and sulfur motifs). In particular, DHBA catechol is going various transformation under these conditions.53 To be noted, upon developing general method, these limitations may result in searching for different examples (e.g. proteins to be compatible) rather than diving into specific solution. The particular behavior of insulin as a molecule, found to generate narrow range in which the Lys pKa is shifted towards lower values.42 Taking advantage of these findings, we have developed a protocol to site specific modify B29 under physiological pH (~7), compatible with sensitive molecules. Labeled insulin was dissolved first in DMF and further diluted with sodium bicarbonate buffer pH~9 and CH3CN (1:1) to be then adjusted (0.5 mM). A 3 equiv. of activated protected Cys was then reacted to allow specific conversion within 4 h. exposure to 95% TFA resulted in the free thiolated form (Figure 4).

Figure 4. LCMS traces for subsequent selective insulin modification at K29. a. DMF, sodium bicarbonate buffer/CH3CN (1:1) (protein concentration=0.5 mM), 3 equiv. of activated protected Cys, 4 h, RT.
The third precise modification on the thiolated labeled K29 then has been attempted employing DiPyBA, 4. Despite the extensive studies and numerous reports about thio-Michael addition protocols, none of the conditions could be useful, mainly due to intrinsic aggregation prone and reactivity versatile (Table 1, SI). A possible reason for the challenges associated with performing the reaction could be found in the presence of unpaired/capped sulfur. This sulfur moiety can potentially serve as intramolecular reducing agent to initiate disulfide scrambling. Moreover, intermolecular disulfide formation to form insoluble aggregates upon homodimerization could be hypothesized. The reaction conditions have an important effect of such competing directions and sulfur reactivity. Upon performing this conjugation reaction in pure water, for 1h, RT the desired product has been obtained (Figure 5A, SI).
Table 1, comparison for insulin DiPyBA conjugation under different solvents

| Entry | Solvent | Conversion(%) |
| 1 | 0.1 M citric acid buffer, pH 5 | – |
| 2 | bicarbonate buffer, pH 7 | – |
| 3 | bicarbonate buffer, pH 9 | – |
| 4 | 6 M HCl_then bicarbonate, pH 7 | – |
| 5 | Water | 100 |
Activity examination
The potency of the novel created GRI 1 was evaluated in cells. The ability to induce AKT protein phosphorylation within cellular environment is an established approach to measure insulin activity.54 Briefly, elevated endogenous levels of AKT serine (Ser) 473 phosphorylation are biomarkers for insulin activity. Endogenous levels of pAKT were measured in a human insulin receptor overexpressed NIH 3T3 cell line, derived from IGF-1R knockout mice. Cells were cultured in DMEM with 10% fetal bovine serum, 100 U/mL penicillin–streptomycin, 2 mg/mL puromycin, and 1 mg/mL normocinat 37 oC under 5% CO2. For the assay, 30,000 cells per well and 100 μL per well, have been plated in a 96-well plate with culture media containing 1% FBS. 20 h later, the media has been removed and 50 μL of culture media with different concentrations of native insulin or the novel GRI have been pipetted into each well. After treatment at 37 oC for 30 min, the solution has been removed and the HTRF pAKT Ser473 kit has been used to measure the phosphorylation of AKT (see SI). The results have shown that the activity of the modified insulin was retained with slight reduction as anticipated. Interestingly, upon examination of glucose activity dependence, it has been found that the presence of the DiPyBA motif and the catechol elements are not interfered with the in vitro activities whether glucose has been applied or not. Surprisingly, the presence of fructose did not affect its activity in a similar manner. With these interesting results in hands, the unique features of internal boronate-catchcol interaction are found to be tolerated with insulin activity. We ask ourselves whether we found an unprecedent narrow direction to benefit from these two molecules for inhibiting insulin fibrillation, without rewiring its responsiveness. The DiPyBA-cathechol potentially would serve as reversible bridge to enhance insulin stability, while improving its overall solubility, upon rescuing it from hydrophobic collapse. As fibrillation assumes to be governed by hydrophobic collapse, we envision such proof of concept de novo design to serve as potentially biology-invisible mask to rescue insulin from its aggregation fate.

Figure5. De novo insulin analogue 1, MS characterization and pAKT assay results in the absence (grey) and the presence of glucose (orange) or fructose (green). Experiments in the assay were performed in quadruplicates.
We next wanted to examine whether the insertion of an additional glucose mimic element would affect the activity and/or the sugar binding capabilities. Therefore, based on our developed PSM concept we exposed recombinant h-insulin (1 mM) to 8 equiv. DHBA over 48h, in 0.1 M Citric acid buffer, pH 5. It was also found that heating to 37oC resulted in shortening the reaction time to 12 h, furnishing with di-modified DHBA at B1 position. The isolated di-modified product was then redissolved in sodium bicarbonate buffer/ACN (1:1), pH~7 and exposed to 3 equiv. Boc-Cys(Trt)-OSu. Subsequently the Boc and Trt groups were removed via treatment with TFA:TIPS:H2O cocktail (95:2.5:2.5), and the product was precipitate in cold ether. Without further purification, the isolated product has been reacted with DiPyBA in neat water to obtain the final tetra-modified insulin bearing two catechol (Figure 6A).
The in vitro activity of the de novo insulin analogue 2 has been examined applying the mentioned above AKT phosphorylation assay (Figure 6B, SI). Our findings show an intact activity of the modified insulin. Upon exposure to glucose and fructose the activity remained stable (Figure 6B).
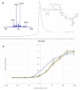
Figure 6. De novo insulin analogue 2, MS characterization and pAKT assay results in the absence (orange) and the presence of glucose (grey) or fructose (green). Experiments in the assay were performed in quadruplicates.
Conclusions
Inspired by the challenges for performing multiplexed insulin site-selective modification, we introduced a different conceptual idea to convene efforts towards the exploration of one protein rather than developing a general protocol. This point of view, potentially would transform the science of proteins if would be utilized. It’s commonly known that attempts are directed to develop general strategies to be useful for wide as possible targets. Nevertheless, despite the importance of such studies, the search for generality has potential to lose valuable information regarding specific targets. Up to date, upon developing a new protocol, when examples are found as not appropriate due to unique properties, potentially other examples would be examined, to demonstrate wide as possible scope. This suggested paradigm change, should pave the way for the young generation of scientists to invest time and resources in developing synthetic tools for the study of particular families of proteins, or proteins, rather than focus on generality (all peptide and proteins). Laying the foundation for this vision, we set out to investigate insulin for specific modification. We have focused our attention on a beneficial therapeutic advantage towards future development of PBA smart insulin. Our findings suggest a synthetic avenue to perform multiple insulin modification precisely with sensitive molecules and without enzymatic engagement. The introduced chemistry and its simplicity have the immediate potential to allow scaling up and manufacturing. We have also shown that attachment of diboronate motif to insulin at K29 did not interfere with its activity in cellular context in the presence and absence of sugars. This interesting finding paves the way for yet to be explored chemistry employing diboronate for reducing fibrillation and subsequent chemistry such as Suzuki cross coupling, cell permeability and other applicationss.55 We believe that this information will be not only useful for possible general cases upon optimization but will enable the profession of protein specific investigator to shed deeper light on the mystery of life. We envision the future exploration of our two novel modified insulin analogues for tackling insulin fibrillation problem, developing slow released formulations and for the development of new GRI based therapy. Moreover, this interesting chemistry and the DiPyBA- catechol internal interaction could be further implemented and extended to other than insulin scaffolds such as inulin likes and insulin mimetics.
Acknowledgement
We thank Stanford Diabetes Research Center and Feasibility Grant for funding support. We thank our lab technician Ms. Terra Lin for her assistance with the pAKT activity assay. We thank an MSc visitor student Ms. Paulina Hegyiova for her assistance. Mr. Maxwell. J. Austin has been acknowledged for his assistance in graphical editing. Dr. Shay Laps is acknowledging Breakthrough T1D (formerly JDRF) foundation for awarding him the 2025-2028 prestigious fellowship for his postdoctoral research at the Chou’s lab-Stanford university, to continue explore de novo smart insulin development with aims to transform blood glucose control therapy in T1D patients.
References
- Karas, J. A., Wade, J. D., Hossain, M. A. The chemical synthesis of insulin: an enduring challenge. Chem. Rev. 121, 4531–4560 (2021).
- Katsoyannis, P. G. Synthesis of Insulin. Science154, 1509-1514 (1966).
- Dixon, G. H., Wardlaw, A. C. Regeneration of insulin activity from the separated and inactive a and b chains. Nature 188, 721–724 (1960).
- Owens, D. R. New horizons-alternative routes for insulin therapy. Nat.Rev.Drug Discov. 1, 529–540 (2002).
- Pedersen-bjergaard, U., Thorsteinsson, B. Reporting severe hypoglycemia in type 1 diabetes: facts and pitfalls. Curr Diab Rep 17, 1–11 (2017).
- Tamborlane, W. V. etal. Continuous Glucose Monitoring and Intensive Treatment of Type 1 Diabetes. N Engl J Med 359, 1464–1476 (2008).
- Lin, N. P., Chou, D. H. C. Modifying insulin to improve performance. Science376, 1270–1271 (2022).
- Maki, Y. etal.Rapid synthesis of glycosylated insulins by flow-based peptide synthesis. Chem. Sci. (2025) doi:10.1039/d5sc01670c.
- Hossain, M. A., Wade, J. D. Novel methods for the chemical synthesis of insulin superfamily peptides and of analogues containing disulfide isosteres. Acc.Chem.Res. 50, 2116–2127 (2017).
- Chen. H. etal.Chemical modification of insulin using flow chemistry. ChemBioChem 25, e202400534 (2024).
- Jarosinski, M. A., Dhayalan, B. Weiss, M. A. ‘Smart’ insulin-delivery technologies and intrinsic glucose-responsive insulin analogues. Diabetologia64, 1016–1029 (2021).
- Chen, Y. et al. Insertion of a synthetic switch into insulin provides metabolite- dependent regulation of hormone-receptor activation. Proc.Natl.Acad.Sci.U.S.A. 118, e2103518118 (2021).
- Hoeg-jensen, T., Havelund, S., Nielsen, P. K. Markussen, J. Reversible insulin self- assembly under carbohydrate control. J. Am. Chem. Soc. 127, 6158–6159 (2005).
- Hoeg-Jensen, T. etal.Glucose-sensitive insulin with attenuation of hypoglycaemia. Nature634, (2024).
- Bakh, N. A. etal.Glucose-responsive insulin by molecular and physical design. Nat. Chem. 9, 937–943 (2017).
- Mannerstedt, K. etal.An aldehyde responsive , cleavable linker for glucose responsive insulins. Chem. Eur.J. 27, 3166–3176 (2021).
- Wu, Q., Wang, L., Yu, H., Wang, J. Chen, Z. Organization of glucose-responsive systems and their properties. Chem. Rev. 111, 7855–7875 (2011).
- Shen, D. etal.Recent progress in design and preparation of glucose-responsive insulin delivery systems. J. Control. Release 321, 236–258 (2020).
- Mo, R., Jiang, T., Di, J. Gu, Z. Emerging micro- and nanotechnology based synthetic approaches for insulin delivery. Chem. Soc. Rev. 43, 3595–3629 (2014).
- O’hagan, M. P. etal.Photocleavable ortho-nitrobenzyl-protected DNA architectures and their applications. Chem. Rev. 123, 6839–6887 (2023).
- Fischer, A. et al. Triggered release of loads from microcapsule-in-microcapsule hydrogel microcarriers: en-route to an “artificial pancreas”. J.Am.Chem.Soc.142, 4223–4234 (2020).
- Gu, Z. etal.Injectable Nano-network for glucose-mediated insulin delivery. ACS Nano 7, 4194–4201 (2013).
- Wang, C. etal.Recent advances in phenylboronic acid-based gels with potential for self-regulated drug delivery. Mol. 2019, 24, 1089 (2019).
- Muttenthaler, M., King, G. F., Adams, D. J. Alewood, P. F. Trends in peptide drug discovery. Nat. Rev. Drug Discov. 20, 309–325 (2021).
- Wu, X. etal.Selective sensing of saccharides using simple boronic acids and their aggregates. Chem.Soc.Rev. 42, 8032–8048 (2013).
- Xiang, Y. etal.Diboronate crosslinking: introducing glucose specificity in glucose- responsive dynamic-covalent networks. J. Control. Release 348, 601–611 (2022).
- Chou, D. H. C. etal.Glucose-responsive insulin activity by covalent modification with aliphatic phenylboronic acid conjugates. Proc. Natl. Acad. Sci. U. S. A. 112, 2401–2406 (2015).
- Lee, H. G., Lautrette, G., Pentelute, B. L. Buchwald, S. L. Palladium-mediated arylation of lysine in unprotected peptides. Angew.Chemie.Int.Ed.56, 3177–3181 (2017).
- Zhao, Z., Laps, S., Gichtin, J. S. Metanis, N. Selenium chemistry for spatio-selective peptide and protein functionalization. Nat. Rev. Chem. 8, 211–229 (2024).
- Laps, S., Satish, G. Brik, A. Harnessing the power of transition metals in solid-phase peptide synthesis and key steps in the (semi)synthesis of proteins. Chem.Soc.Rev.50, 2367–2387 (2021).
- Canovas, C., etal.Site-specific dual labeling of proteins on cysteine residues with chlorotetrazines. Angew. Chemie. Int. Ed. 57, 10646–10650 (2018).
- Vinogradova, E., etal. Arylation chemistry for bioconjugation. Angew.ChemieInt. Ed. 58, 4810- 4839 (2019).
- Jbara, M. etal.Palladium prompted on-demand cysteine chemistry for the synthesis of challenging and uniquely modified proteins. Nat. Commun. 9, 3154 (2018).
- Zhang, C. etal.π-Clamp-mediated cysteine conjugation. Nat.Chem.8, 120–128 (2016).
- Willwacher, J., Raj, R., Mohammed, S., Davis, B. G. Selective metal-site-guided arylation of proteins. J. Am. Chem. Soc. 138, 8678–8681 (2016).
- Vinogradova, E. V., Zhang, C., Spokoyny, A. M., Pentelute, B. L. Buchwald, S. L. Organometallic palladium reagents for cysteine bioconjugation. Nature526, 687–691 (2015).
- Zhao, Z., Shimon, D. Metanis, N. Chemoselective copper-mediated modification of selenocysteines in peptides and proteins. J.Am.Chem.Soc.2021,143, 12817–12824 (2021).
- Laps, S. Metanis, N. Organic solvent enhances oxidative folding of disulfide-rich proteins. Nat. Chem. 16, 680–681 (2024).
- Laps, S., etal. General synthetic strategy for regioselective ultrafast formation of disulfide bonds in peptides and proteins. Nat. Commun. 12, 870 (2021).
- Dardashti, R. N., Laps, S., Gichtin, J. S., Metanis, N. The semisynthesis of nucleolar human. Chem. Sci. 14, 12723–12729 (2023).
- Shi, X. etal.Reversible stapling of unprotected peptides via chemoselective methionine bis-alkylation/dealkylation. Chem. Sci. 9, 3227–3232 (2018).
- Chan, Y., Oda, G. Kaplan, H. Chemical properties of the functional groups of insulin. Biochem.J.193, 419–425 (1981).
- Fryszkowska. A., etal. A chemoenzymatic strategy for site-selective functionalization of native peptides and proteins. Science 376, 1321-1327 (2022).
- Mousa, R., etal. Oxidative protein folding. Commun.Chem.4, 30 (2021).
- Zhang, Y., et al. Cyclization of the analgesic α-conotoxin vc1.1 with a non-natural linker: effects on structure, stability, and bioactivity. J. Pept. Sci. 31, (2025).
- Kam, A., etal.Ultrafast biomimetic oxidative folding of cysteine-rich peptides and microproteins in organic solvents. Angew. Chem. Int. Ed. 63, e202317789 (2024).
- Avital-Shmilovici, M., et al. Fully convergent chemical synthesis of ester insulin: determination of the high resolution x-ray structure by racemic protein crystallography. J. Am. Chem. Soc. 135, 3173–3185 (2013).
- Tombling, B. J., Wang, C. K. & Craik, D. J. EGF-like and other disulfide-rich microdomains as therapeutic scaffolds. Angew.Chem.Int.Ed.59, 11218–11232 (2020).
- Laps, S. etal.Insight on the order of regioselective ultrafast formation of disulfide bonds in (antimicrobial) peptides and miniproteins. Angew. Chemie. Int. Ed. 60, 24137–24143 (2021).
- Park, J. etal.Activation of the insulin receptor by an insulin mimetic peptide. Nat. Commun. 13, (2022).
- Kirk, N. S. etal.Activation of the human insulin receptor by non-insulin-related peptides. Nat. Commun. 13, 5695 (2022).
- Isidro-Llobet, A., Álvarez, M., Albericio, F. Amino acid-protecting groups. Chem. Rev. 109, 2455–2504 (2009).
- Maier, G. P., Bernt, C. M., Butler, A. Catechol oxidation: considerations in the design of wet adhesive materials. Biomater. Sci. 6, 332–339 (2018).
- lin, N. P., etal. synthesis and characterization of phenylboronic acid-modified insulin with glucose-dependent solubility. Front. Chem. 10, 859133 (2022).
- Ghosh, P., Seitz, O. Boronic acid-linked apo-zinc finger protein for ubiquitin delivery in live cells. ChemBioChem 26, e202401040 (2025).






