Rowan Finch
Northwood University
Abstract
The placebo effect, once dismissed as a nuisance variable in clinical trials, is now recognized as a genuine and potent psychobiological phenomenon. This comprehensive review traces the evolution of this concept, synthesizing historical, mechanistic, ethical, and clinical research. The paper is structured in three parts. First, it examines the foundation, reviewing how the placebo effect was historically conceptualized as a source of bias in randomized controlled trials (RCTs) post-1948 and how its formal definition has evolved from denoting an inert substance to representing the complex therapeutic context. Second, it delves into the core debate and latest research, summarizing recent findings on the neurobiological mechanisms underpinning placebo and nocebo effects—with a focus on pain and Parkinson’s disease—and reviewing the distinct roles of psychological drivers like expectation and classical conditioning. Finally, the paper explores the implications and future of placebo science by analyzing the ethical frameworks, guided by Principlism, for its clinical application and detailing evidence-based, ethically sound strategies to harness the placebo effect to enhance therapeutic outcomes. By integrating these perspectives, this review charts the journey of the placebo effect from a statistical artifact to be eliminated to a powerful aspect of healing that can be ethically leveraged in modern clinical practice.
Keywords: placebo effect, nocebo effect, expectation, classical conditioning, neurobiology, randomized controlled trial (RCT), nuisance variable, bioethics, principlism, open-label placebo, therapeutic context
Introduction
The placebo effect is one of the most fascinating and challenging phenomena in medicine and psychology. It represents the measurable, observable, or felt improvement in health not attributable to an active treatment’s specific properties. For decades, it was relegated to the sidelines of scientific inquiry—a statistical ghost in the machine of the randomized controlled trial (RCT), a “nuisance variable” that needed to be isolated and subtracted to reveal the “true”
efficacy of a new drug or therapy. However, a profound conceptual shift has occurred over the last several decades. The placebo effect is no longer seen as an illusion but as a powerful demonstration of the intricate connection between mind and body, mediated by specific and measurable neurobiological pathways.
This review paper provides a comprehensive synthesis of this evolution. It charts the journey of the placebo effect from its historical framing to its current status as a legitimate and powerful therapeutic agent. The paper follows a logical progression, beginning with the historical foundations that shaped modern clinical research, moving to the cutting-edge science of its underlying mechanisms, and concluding with the practical and ethical considerations of harnessing its power in clinical practice. By connecting these diverse domains, this review aims to provide a cohesive understanding of one of the most fundamental aspects of the healing process.
Part I: The Foundation – From Nuisance to Phenomenon
The modern understanding of the placebo effect is built upon a historical foundation where it was primarily seen as an obstacle to scientific clarity. This section reviews how this initial framing shaped the gold standard of clinical research and how, over time, the very definition of the placebo effect was forced to evolve in response to growing scientific evidence.
The Placebo Effect as a ‘Nuisance Variable’ in Randomized Controlled Trials
The placebo effect has played a pivotal role in the evolution of RCTs in both medicine and psychology. From the landmark 1948 British Medical Research Council (MRC) streptomycin trial onward, it was increasingly conceptualized as a “nuisance variable”: a source of bias that could obscure the true efficacy of interventions (Atlas & Wager, 2009; Bentin et al., 2024; Ho, 2023). This framing drove methodological innovations, particularly the adoption of double-blinding and inert placebos, to isolate specific treatment effects from non-specific placebo responses.
This conceptualization was rooted in the concern that factors like patient expectations or provider behavior could inflate estimates of treatment efficacy. This led to a methodological imperative to “control away” these effects through rigorous trial design (Ho, 2023; Bentin et al., 2024). This need to distinguish pharmacological effects from those produced by contextual factors heavily influenced the design of control groups, ensuring that observed outcomes could be attributed to the intervention under investigation (Meissner et al., 2011). Empirical studies reinforced this need; for instance, in a double-blind sham surgery trial for Parkinson’s disease, patients’ perceived treatment assignment had a significant impact on quality of life outcomes, regardless of the actual intervention received (McRae et al., 2004). Similarly, placebo response rates in conditions like PTSD were found to range from 19% to 62%, underscoring the substantial influence of non-specific factors (Benedetti et al., 2018).
This led to the standardization of several key methodological strategies, summarized in Table 1.

These strategies became standard after the recognition that even well-designed active-control trials could not reliably demonstrate efficacy without evidence that the active treatment consistently outperformed a placebo—a property known as “assay sensitivity” (Temple & Ellenberg, 2000). While this nuisance-variable framing drove methodological rigor, it also sparked ongoing ethical debates, particularly regarding the use of placebos when effective treatments already existed and the need for clear informed consent (Niazi, 2024; Temple & Ellenberg, 2000).
The Evolution of the Placebo Effect’s Definition
The historical focus on controlling for the placebo effect was predicated on a narrow definition: the effect of an inert substance. However, beginning in the late 20th century, this definition proved insufficient. Advances in neurobiology and psychology revealed that placebo responses were not merely an absence of an active ingredient but the presence of a genuine psychobiological phenomenon, forcing a profound evolution in its formal scientific definition (Bentin et al., 2024; Koshi & Short, 2007; Peiris et al., 2018).
The conceptualization has shifted from a focus on the placebo object (the inert pill) to the patient’s response to the overall therapeutic context. This evolution can be traced across several decades, as outlined in Table 2.


This shift was driven by key scientific insights. Psychological models highlighted the central roles of expectancy (a patient’s belief in a treatment’s efficacy) and classical conditioning (learned associations between treatment cues and outcomes) (Meissner et al., 2011; Peiris et al., 2018). Concurrently, neuroimaging studies in the 2000s provided the biological evidence, demonstrating that placebo administration could activate specific brain regions, such as the ventromedial prefrontal cortex (vmPFC), insula, and amygdala, and trigger the release of endogenous neurotransmitters (Geuter et al., 2017; Girach et al., 2019).
This led to the modern, integrated understanding of the placebo effect as a “meaning response,” where the change in a patient’s perception of their illness and its treatment can drive tangible physiological changes (Brody, 2018). This redefinition legitimized the study of the placebo effect as a phenomenon in its own right, moving it from a mere methodological control to a potential therapeutic tool.
Part II: The Core Debate & Latest Research
With the placebo effect established as a real psychobiological phenomenon, research in the 21st century has focused on dissecting its precise mechanisms. This section delves into the “how” and “why” of placebo responses, reviewing the latest findings on its neurobiological hardware and the psychological software that activates it.
Neurobiological Mechanisms of Placebo and Nocebo Effects
In the last five years, research has rapidly advanced our understanding of the neurobiological mechanisms underlying both positive (placebo) and negative (nocebo) health outcomes driven by patient expectations. Pain and Parkinson’s disease (PD) have served as powerful model systems for these investigations, with findings beginning to be generalized to other conditions like depression, anxiety, and immune responses (Frisaldi et al., 2023; Petrie & Rief, 2019; Rossettini et al., 2023).
Research has identified key neurotransmitter systems and brain circuits. In pain research,
placebo analgesia in healthy participants is robustly mediated by the endogenous opioid system
and the endocannabinoid system (Skyt et al., 2019). However, this opioid involvement is
surprisingly limited or absent in patients with chronic pain, suggesting that long-term pain may
alter or impair this pathway (Frisaldi et al., 2023; Skyt et al., 2019). In contrast, nocebo
hyperalgesia (increased pain) is linked to the cholecystokinergic (CCK) system. In PD, the
dopaminergic system is central, as placebos can trigger the release of dopamine in the striatum,
leading to objective improvements in motor symptoms (Frisaldi et al., 2023).
Specific brain circuits are also consistently implicated. The prefrontal cortex is a critical hub for
expectation, anticipation, and cognitive modulation of placebo responses across conditions. The
integrity of descending pain control pathways (white matter tracts connecting the brainstem and
spinal cord) correlates with an individual’s responsiveness to placebo analgesia (Frisaldi et al.,
2023).
These effects are not uniform and are influenced by individual differences. For instance, some
studies suggest males show stronger placebo effects while females exhibit more pronounced
nocebo responses, potentially due to differences in stress processing and opioid transmission
(Frisaldi et al., 2023). The magnitude of these neurobiological effects is summarized in Table 3.
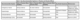
While the mechanisms in pain and PD are increasingly well-defined, their generalization to
conditions like depression, anxiety, and immune disorders is an active area of research. It is
hypothesized that disruption of prefrontal-mediated expectation processes may impair placebo
responsiveness in these disorders, but direct evidence remains limited (Frisaldi et al., 2023).
Psychological Mechanisms: The Roles of Expectation andClassical Conditioning
The neurobiological cascades described above are not spontaneous; they are triggered by
psychological processes. The two primary psychological mechanisms that initiate and shape
placebo responses are expectation (a conscious belief that a treatment will work) and classical
conditioning (a learned, often unconscious, association between a neutral stimulus and an
active one).
Expectation has been shown to be a powerful and direct driver of placebo effects. In a large
study of over 2,000 chronic pain patients, higher pretreatment expectations predicted greater
improvements in pain intensity, depressive symptoms, and treatment satisfaction six months
later (Cormier et al., 2016). The neurobiological link is clear in PD, where placebo-induced
dopamine release in the striatum was only observed when patients were told there was a 75%
chance of receiving the active drug, suggesting an optimal level of expectation or anticipation is
required to trigger a biological response (Lidstone et al., 2010). Across pain and depression,
meta-analyses suggest that positive expectations can account for up to 70% of the overall
treatment effect (Bingel, 2020).
Classical conditioning represents a distinct, learning-based pathway. It occurs when a neutral
cue (e.g., a saline injection) is repeatedly paired with an active drug (e.g., morphine). After
several pairings, the neutral cue alone can elicit the drug’s effect (e.g., analgesia). Crucially,
studies have demonstrated that conditioning can induce placebo analgesia even without
conscious expectation or awareness, for instance, by using hidden or subliminal cues (Bąbel,
2019; Stewart-Williams & Podd, 2004). This mechanism appears particularly potent in children,
who show strong placebo hypoalgesia from conditioning, especially when the initial learning
experience involves a noticeable reduction in pain (Gniß et al., 2020).
While often presented as separate, these two mechanisms interact. Prior experiences
(conditioning) are a primary source for forming future beliefs (expectations). However, the
evidence for conditioning as a truly independent, non-cognitive pathway is strongest in pain
research (Bąbel, 2019). The distinct and overlapping contributions are summarized in Table 4.


Understanding these psychological triggers is paramount, as they represent the levers that can
be pulled in a clinical setting to activate the beneficial neurobiological responses of the placebo
effect.
Part III: Implications & Future – From Science to Practice
The scientific validation of the placebo effect and the elucidation of its mechanisms inevitably
lead to a critical question: “So what?” If placebo effects are real, powerful, and predictable, how
can this knowledge be responsibly translated into clinical practice? This final section addresses
the crucial ethical frameworks required for its use and reviews the evidence-based strategies for
ethically harnessing its power to improve patient care.
Ethical Frameworks for the Clinical Application of Placebos
The use of placebos in clinical practice, particularly in pain management and psychiatry,
presents complex ethical challenges. The evaluation of these challenges in Western bioethics is
primarily guided by Principlism, which balances four core principles: autonomy (respect for a
patient’s right to make informed decisions), beneficence (acting in the patient’s best interest),
non-maleficence (doing no harm), and justice (fair distribution of benefits and risks).
The central ethical conflict has historically revolved around the use of deceptive placebos (also
known as “pure” placebos), where a patient is given an inert substance without their knowledge.
This practice creates a direct clash between principles. While it may be intended to produce a
therapeutic benefit (beneficence), it fundamentally violates patient autonomy by subverting
informed consent and involves active deception (Annoni & Miller, 2016). Furthermore, it risks
significant harm (non-maleficence) if the patient discovers the deception, which can irreparably
damage the patient-provider relationship and trust in the medical system.
In response to this ethical dilemma, recent years have seen the rise of open-label placebos
(OLPs), where patients are transparently informed that they are receiving a substance with no
active pharmacological ingredient, along with a rationale that belief and expectation can
modulate symptoms. This approach has proven to be a promising solution, as it attempts to harness the placebo effect while upholding ethical principles. Table 5 provides an ethical
evaluation of both approaches through the lens of Principlism.

Studies have shown that OLPs are effective. In trials for chronic pain and psychiatric disorders,
OLPs produce significant improvements in symptoms compared to no treatment (Bschor et al.,
2024; Kaptchuk et al., 2020; Kube et al., 2019). While patients are sometimes skeptical, they
consistently value the transparency and honesty of the OLP approach (Locher et al., 2021).
Thus, OLPs largely resolve the core ethical conflict of deceptive placebo use by aligning the
goal of therapeutic benefit with the fundamental duty to respect patient autonomy.
Strategies for Ethically Harnessing the Placebo Effect in Clinical Practice
Building on a foundation of scientific understanding and ethical principles, a toolkit of practical,
evidence-based strategies has emerged for harnessing the placebo effect to enhance patient
outcomes. These strategies move beyond the prescription of inert pills and focus on optimizing the entire therapeutic context. They can be categorized as psychological, communicative,
procedural, and pharmacological.
Psychological and Communicative Strategies are the cornerstone of leveraging the placebo
effect. This involves actively fostering positive—but realistic—patient expectations. A provider’s
communication style is critical; demonstrating warmth, competence, and empathy has been
shown to amplify the physiological impact of positive expectations, leading to greater reduction
in symptoms like an allergic response (Howe et al., 2017).
Procedural Strategies focus on the environment and rituals of care. As discussed, the use of
open-label placebos is a primary, ethically sound procedural strategy, with RCTs demonstrating
significant reductions in pain intensity, functional disability, and depression scores in patients
with chronic back pain compared to treatment as usual (Kleine-Borgmann et al., 2019). Another
powerful procedural strategy is facilitating social observational learning, where observing
positive treatment outcomes in other patients has been shown to augment placebo analgesia in
those with chronic pain (Schwartz et al., 2021).
Pharmacological Strategies are more nuanced. Rather than using drugs to create placebo
effects, this involves leveraging knowledge of conditioning to optimize active treatments. For
example, conditioning principles could be used to design dose-reduction or dose-extension
schedules, where an active drug’s effect is maintained with fewer doses or lower dosages over
time by pairing it with a placebo, thus reducing side effects and costs (Colloca, 2019).
The effectiveness of these ethical, non-deceptive strategies is supported by evidence measuring
both patient-reported outcomes (e.g., pain scores, quality of life) and objective clinical markers
(e.g., inflammatory markers, medication usage, physiological readings), confirming they produce
genuine psychobiological changes (Zubieta & Stohler, 2009). Table 6 summarizes these
actionable strategies.


By systematically applying these evidence-based strategies, clinicians can maximize
therapeutic benefit from both active treatments and the inherent healing power of the placebo
effect, all within a robust ethical framework.
Conclusion
The concept of the placebo effect has completed a remarkable journey—from a statistical
nuisance in the mid-20th century to a central topic of neuroscientific and clinical investigation
today. The historical need to control for the placebo effect in RCTs forged the gold standard of
modern clinical trials, but the very success of that methodology revealed a phenomenon too
powerful to ignore. The definition has expanded from an inert pill to the entire psychosocial
context of healing, driven by concrete psychological mechanisms like expectation and
conditioning and substantiated by measurable changes in brain circuitry and neurochemistry.
This deeper understanding has shifted the conversation from one of elimination to one of ethical
application. The central conflict of deceptive placebo use, which pits beneficence against
autonomy, is being resolved through transparent approaches like open-label placebos. A new
clinical paradigm is emerging, one that recognizes the power of the therapeutic encounter itself.
By optimizing communication, managing expectations, and structuring clinical rituals,
practitioners can ethically and effectively activate the same endogenous healing pathways that
the placebo effect so powerfully demonstrates. The future of placebo research lies not in finding
better placebos, but in better understanding and cultivating the patient’s innate capacity to heal,
transforming every clinical interaction into an opportunity to enhance therapeutic outcomes.
References
Annoni, M., & Miller, F. G. (2016). Placebo effects and the ethics of therapeutic communication: A pragmatic perspective. Kennedy Institute of Ethics Journal, 26(1), 79–103. https://doi.org/10.1353/ken.2016.0004
Atlas, L. Y., & Wager, T. D. (2009). Placebo effects. In Handbook of Neuroscience for the Behavioral Sciences (1st ed.). Wiley. https://doi.org/10.1002/9780470478509.neubb002063
Bąbel, P. (2019). Classical conditioning as a distinct mechanism of placebo effects. Frontiers in Psychiatry, 10. https://doi.org/10.3389/fpsyt.2019.00449
Benedetti, F., Piedimonte, A., & Frisaldi, E. (2018). How do placebos work? European Journal of Psychotraumatology, 9(sup3). https://doi.org/10.1080/20008198.2018.1533370
Bentin, J. M., Arendt-Nielsen, L., & Bihlet, A. R. (2024). Placebo effect in clinical practice. Ugeskrift for Læger, 1–8. https://doi.org/10.61409/v02240121
Bingel, U. (2020). Placebo 2.0: The impact of expectations on analgesic treatment outcome. Pain, 161(Supplement 1), S48–S56. https://doi.org/10.1097/j.pain.0000000000001981
Brody, H. (2018). Meaning and an overview of the placebo effect. Perspectives in Biology and Medicine, 61(3), 353–360. https://doi.org/10.1353/pbm.2018.0048
Bschor, T., Nagel, L., Unger, J., Schwarzer, G., & Baethge, C. (2024). Differential outcomes of placebo treatment across 9 psychiatric disorders. JAMA Psychiatry, 81(8), 757. https://doi.org/10.1001/jamapsychiatry.2024.0994
Colloca, L. (2019). The placebo effect in pain therapies. Annual Review of Pharmacology and Toxicology, 59(1), 191–211. https://doi.org/10.1146/annurev-pharmtox-010818-021542
Cormier, S., Lavigne, G. L., Choinière, M., & Rainville, P. (2016). Expectations predict chronic pain treatment outcomes. Pain, 157(2), 329–338. https://doi.org/10.1097/j.pain.0000000000000379
Frisaldi, E., Shaibani, A., Benedetti, F., & Pagnini, F. (2023). Placebo and nocebo effects and mechanisms associated with pharmacological interventions: An umbrella review. BMJ Open, 13(10), e077243. https://doi.org/10.1136/bmjopen-2023-077243
Geuter, S., Koban, L., & Wager, T. D. (2017). The cognitive neuroscience of placebo effects: Concepts, predictions, and physiology. Annual Review of Neuroscience, 40(1), 167–188. https://doi.org/10.1146/annurev-neuro-072116-031132
Girach, A., Aamir, A., & Zis, P. (2019). The neurobiology under the placebo effect. Drugs of Today, 55(7), 469. https://doi.org/10.1358/dot.2019.55.7.3010575
Gniß, S., Kappesser, J., & Hermann, C. (2020). Placebo effect in children: The role of expectation and learning. Pain, 161(6), 1191–1201. https://doi.org/10.1097/j.pain.0000000000001811
Hardman, D. (2024). A fictionalist account of open-label placebo. The Journal of Medicine and Philosophy: A Forum for Bioethics and Philosophy of Medicine, 49(3), 246–256. https://doi.org/10.1093/jmp/jhae008
Ho, D. (2023). What placebos teach us about health and care (1st ed.). Cambridge University Press. https://doi.org/10.1017/9781009085496
Howe, L. C., Goyer, J. P., & Crum, A. J. (2017). Harnessing the placebo effect: Exploring the influence of physician characteristics on placebo response. Health Psychology, 36(11), 1074–1082. https://doi.org/10.1037/hea0000499
Howick, J., & Hoffmann, T. (2018). How placebo characteristics can influence estimates of intervention effects in trials. Canadian Medical Association Journal, 190(30), E908–E911. https://doi.org/10.1503/cmaj.171400
Kaptchuk, T. J., Hemond, C. C., & Miller, F. G. (2020). Placebos in chronic pain: Evidence, theory, ethics, and use in clinical practice. BMJ, m1668. https://doi.org/10.1136/bmj.m1668
Kleine-Borgmann, J., Schmidt, K., Hellmann, A., & Bingel, U. (2019). Effects of open-label placebo on pain, functional disability, and spine mobility in patients with chronic back pain: A randomized controlled trial. Pain, 160(12), 2891–2897. https://doi.org/10.1097/j.pain.0000000000001683
Koshi, E. B., & Short, C. A. (2007). Placebo theory and its implications for research and clinical practice: A review of the recent literature. Pain Practice, 7(1), 4–20. https://doi.org/10.1111/j.1533-2500.2007.00104.x
Kube, T., Rief, W., Vivell, M.-B., Schäfer, N. L., Vermillion, T., Körfer, K., & Glombiewski, J. A. (2019). Deceptive and nondeceptive placebos to reduce pain. The Clinical Journal of Pain, 36(2), 68–79. https://doi.org/10.1097/ajp.0000000000000781
Lidstone, S. C., Schulzer, M., Dinelle, K., Mak, E., Sossi, V., Ruth, T. J., de la Fuente-Fernández, R., Phillips, A. G., & Stoessl, A. J. (2010). Effects of expectation on placebo-induced dopamine release in Parkinson disease. Archives of General Psychiatry, 67(8), 857. https://doi.org/10.1001/archgenpsychiatry.2010.88
Locher, C., Buergler, S., Frey Nascimento, A., Kost, L., Blease, C., & Gaab, J. (2021). Lay perspectives of the open-label placebo rationale: A qualitative study of participants in an experimental trial. BMJ Open, 11(8), e053346. https://doi.org/10.1136/bmjopen-2021-053346
McRae, C., Cherin, E., Yamazaki, T. G., Diem, G., Vo, A. H., Russell, D., Ellgring, J. H., Fahn, S., Greene, P., Dillon, S., Winfield, H., Bjugstad, K. B., & Freed, C. R. (2004). Effects of perceived treatment on quality of life and medical outcomes in a double-blind placebo surgery trial. Archives of General Psychiatry, 61(4), 412. https://doi.org/10.1001/archpsyc.61.4.412
Meissner, K., Bingel, U., Colloca, L., Wager, T. D., Watson, A., & Flaten, M. A. (2011). The placebo effect: Advances from different methodological approaches. The Journal of Neuroscience, 31(45), 16117–16124. https://doi.org/10.1523/jneurosci.4099-11.2011
Niazi, S. K. (2024). Advice to the FDA to improve its proposed guidelines to rationalize clinical trials by restricting placebo control, preventing low-powered studies, and disallowing studies where bioavailability is not proven. Pharmaceuticals, 17(11), 1424. https://doi.org/10.3390/ph17111424
Peiris, N., Blasini, M., Wright, T., & Colloca, L. (2018). The placebo phenomenon: A narrow focus on psychological models. Perspectives in Biology and Medicine, 61(3), 388–400. https://doi.org/10.1353/pbm.2018.0051
Petrie, K. J., & Rief, W. (2019). Psychobiological mechanisms of placebo and nocebo effects: Pathways to improve treatments and reduce side effects. Annual Review of Psychology, 70(1), 599–625. https://doi.org/10.1146/annurev-psych-010418-102907
Rossettini, G., Campaci, F., Bialosky, J., Huysmans, E., Vase, L., & Carlino, E. (2023). The biology of placebo and nocebo effects on experimental and chronic pain: State of the art. Journal of Clinical Medicine, 12(12), 4113. https://doi.org/10.3390/jcm12124113
Schwartz, M., Fischer, L.-M., Bläute, C., Stork, J., Colloca, L., Zöllner, C., & Klinger, R. (2021). Observing treatment outcomes in other patients can elicit augmented placebo effects on pain treatment: A double-blinded randomized clinical trial with patients with chronic low back pain. Pain, 163(7), 1313–1323. https://doi.org/10.1097/j.pain.0000000000002513
Skyt, I., Lunde, S. J., Baastrup, C., Svensson, P., Jensen, T. S., & Vase, L. (2019). Neurotransmitter systems involved in placebo and nocebo effects in healthy participants and patients with chronic pain: A systematic review. Pain, 161(1), 11–23. https://doi.org/10.1097/j.pain.0000000000001682
Stewart-Williams, S., & Podd, J. (2004). The placebo effect: Dissolving the expectancy versus conditioning debate. Psychological Bulletin, 130(2), 324–340. https://doi.org/10.1037/0033-2909.130.2.324
Temple, R., & Ellenberg, S. S. (2000). Placebo-controlled trials and active-control trials in the evaluation of new treatments. Part 1: Ethical and scientific issues. Annals of Internal Medicine, 133(6), 455–463. https://doi.org/10.7326/0003-4819-133-6-200009190-00014 Zubieta, J., & Stohler, C. S. (2009). Neurobiological mechanisms of placebo responses. Annals of the New York Academy of Sciences, 1156(1), 198–210. https://doi.org/10.1111/j.1749-6632.2009.04424.x





