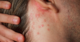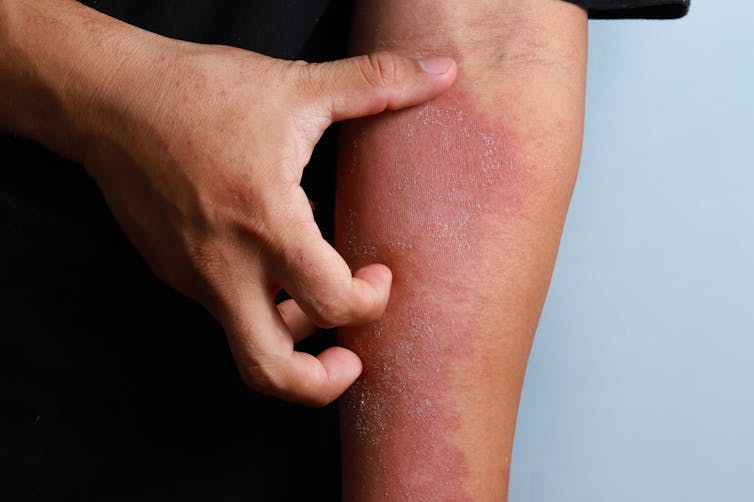
Rashes can be thought of as a dysfunctional community of skin cells. Your skin harbors dozens of distinct cell types, including those that form blood vessels, nerves and the local immune system of the skin. For decades, clinicians have largely been diagnosing rashes by eye. While examining the physical appearance of a skin sample under a microscope may work for more obvious skin conditions, many rashes can be difficult to distinguish from one another.
At the molecular level, however, the differences between rashes become more clear.
Scientists have long known that molecular abnormalities in skin cells cause the redness and scaliness seen in conditions like psoriasis and eczema. While almost all the various cell types in your skin can release chemicals that worsen inflammation, which ones leads to rash formation remains a mystery and may vary from patient to patient.
But molecular testing of skin rashes isn’t a common practice because of technological limitations. Using a new approach, my colleagues and I were able to analyze the genetic profiles of skin rashes and quantitatively diagnose their root causes.
High-res skin profiles
Traditional genetic analyses work by averaging out the activity of thousands of genes across millions of cells.
Genetically testing tissue samples is standard practice for conditions like cancer. Clinicians collect and analyze tumor biopsies from patients to determine a particular cancer’s unique molecular characteristics. This genetic fingerprint helps oncologists predict whether a cancer will spread or which treatments might work best. Cancer cells lend themselves to this form of testing because they often grow into recognizable masses that make them easy to isolate and analyze.
But skin is a complex mixture of cells. Collapsing these unique cell communities into a single group may obscure genetic signatures essential to diagnosis.
Recent technological advances called single-cell RNA sequencing, however, have enabled scientists to preserve the identity of each type of cell that lives in the skin. Instead of averaging the genetic signatures across all cell types in bulk, single-cell RNA sequencing analyses allow each cell to preserve its unique characteristics.
Using this approach, my colleagues and I isolated over 158,000 immune cells from the skin samples of 31 patients. We measured the activity of about 1,000 genes from each of those cells to create detailed molecular fingerprints for each patient. By analyzing these fingerprints, we were able to pinpoint the genetic abnormalities unique to the immune cells residing in each rash type. This allowed us to quantitatively diagnose otherwise visually ambiguous rashes.
We also observed that some patients had treatment responses consistent with what we expected with our predicted diagnoses. This suggests that our concept could viably be expanded for further testing.
To make our approach available to clinicians and scientists, we developed an open source web database called RashX that contains the genetic fingerprints of different rashes. This database will allow clinicians to compare the genetic profile of their patients’ rashes to similar profiles in our database. A closely matching genetic fingerprint might yield clues as to what caused their patient’s rash and lead to potential treatment avenues.
Open source diagnostics
The rapid development of drugs that target the immune system in recent years has inundated doctors with difficult treatment decisions for individual patients. For example, while certain drugs that act on the immune system are known to work well for conditions like psoriasis or eczema, many patients have atypical rashes that can’t be precisely diagnosed.
An open source database like ours could help enable clinicians to profile and diagnose these rashes, providing a stepping stone to choose a suitable treatment.
Furthermore, chronic inflammatory diseases that affect organs other than the skin share similar genetic abnormalities. Lab tests that can illuminate the root causes of skin diseases can likely be expanded to many other conditions.
Our RashX project initially focused on just two very common types of rashes, psoriasis and eczema. It is unknown whether other types of rashes will have similar genetic profiles to psoriasis and eczema or instead have their own unique fingerprints. It is also unclear which parts of the fingerprint would best predict drug response.
But RashX is a living web resource that will grow more useful as more scientists collaborate and contribute new data. Our lab is also working to simplify the process of developing genetic profiles of rashes to make participating in this area of research more accessible for clinics around the world. With more data, we believe that projects like RashX will make precision testing for rashes an essential next step in diagnosis and treatment.
[Understand new developments in science, health and technology, each week. Subscribe to The Conversation’s science newsletter.]Raymond J. Cho, Associate Professor of Dermatology, University of California, San Francisco
This article is republished from The Conversation under a Creative Commons license. Read the original article.