Picture the nearest sponge in a nearby sink as it collects water within itself. The moment the sponge comes into contact with water, the water starts seeping into pores and voids within the sponge. The sponge, as a result of all the additional water now present within it, swells and enlarges in response.
The mental exercise may seem moot at first, but this simple household phenomena is also at play, albeit at a much smaller scale, in this particular new study published in the journal Chem. Here, scientists from Dartmouth College created so-called “porous organic frameworks” (POFs) from elements like carbon (C), oxygen (O), and nitrogen (N).
The new material class is one of the latest products of materials science, which explores how we can manipulate the characterization of the materials that we use in our everyday lives by exploring the interplay between structure, processing, performance, and properties—a concept called the tetrahedron of materials science. Similar explorations into the world of materials science can be found in recent research, from smart materials that may one day help heat and cool buildings to ceramics that can stop and heal its own cracks.
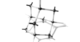
This novel POF was created with the intended function of having the crystal structure “expand” when new molecules gather within its porous internals. Said lead author Chenfeng Ke, Assistant Professor from Dartmouth: “Picture a diamond that behaves like a rubber band.”
Ke and team achieved this feat by making use of bisulfate ions, which function as “soft joints” within the structure of the POF. These clusters of bisulfate ions usually repel each other; however, their interactions with nearby structural components within the POF keep them in place and stop them from repelling each other. However, once a foreign molecule enters the vicinity, the lock that keeps the bisulfites in place is broken, allowing them to repel each other again—thereby “growing” the structure of the materia as it absorbs new materials.
Testing was done by exposing the resultant crystal to a phenol solution, which allowed it to expand up to more than twice its length within 20 minutes; once taken off the phenol solution, the crystal eventually returned to its original size after 10 minutes.
The study itself was headed by research associate Jayanta Samanta, who mentions that the resulting crystals appeared as millimeter-long tiny needles. Ke followed: “Seeing the crystal expand and contract to this extent is remarkable.”
The POF structure may see various potential applications in the future, such as in environmental cleanup and water sanitation cases where absorption of certain types of chemicals are required.
References
- Irving, M. (2021, December 8). Stretchy, porous crystal can grow on demand to trap more molecules. New Atlas. https://newatlas.com/materials/stretchy-crystal-porous-organic-framework/
- Samanta, J., Dorn, R. W., Zhang, W., Jiang, X., Zhang, M., Staples, R. J., Rossini, A. J., & Ke, C. (2021). An ultra-dynamic anion-cluster-based organic framework. Chem, 0(0). https://doi.org/10.1016/j.chempr.2021.11.014