
On 9 January 2023, the United Nations released its latest report on the status of the ozone layer. It highlighted that this protective barrier is on track toward recovery and should be fully restored by the second half of the 21st century.
There is an urgent need for solutions to help limit climate disruption, yet effective decision-making remains challenging. In light of this context, it is worthwhile to recall the scientific saga that led to the signing of the 1987 Montreal Protocol.
Resulting from the outstanding coordinated efforts of scientists, industrialists and policymakers, that text was the first environmental agreement to be unanimously ratified. Today, the treaty and its subsequent amendments are paving the way toward complete elimination of the substances that destroy the ozone layer.
Indispensable ozone
As pointed out by the 2023 annual report, the ozone hole over Antarctica returns every year from September to November. During this three-month period, ozone concentrations falling below 220 Dobson units.
Ozone protects us from solar radiation by absorbing the most powerful ultraviolet rays into the upper reaches of the atmosphere (10 to 50 km above the Earth’s surface). As such, no ozone means no life on Earth.
We have long known which chemical processes produce and destroy this atmospheric compound filling the stratosphere. In fact, ozone measurements have been carried out since the Interwar period (1919–39). But from the 1980s onward, more and more devices for monitoring the ozone have been developed, from ground-based instruments to weather balloons released on a regular basis by meteorological institutes, and satellites that monitor the distribution of ozone worldwide.
Back in the 1980s, scientists were concerned about the impact of supersonic flights and other potential disturbances on this stable atmospheric layer, which is less affected by air masses than those closer to Earth.
A question mark above Antarctica
In 1984, a discovery prompted a radical shift in priorities within the scientific community. Two independent teams – one from Japan, the other from the United States – had reported observing a loss of ozone above the Antarctica each October and this loss was becoming more severe by the year. The first puzzle in their findings was that the loss of concentration was observed in a region of the globe that should have been unaffected by human-induced emissions.
Suspicions were soon cast on chlorinated and compounds, the prime targets being chlorofluorocarbons and halons. In the 1960s, these “magic” industrial compounds became widely available. From then on, they were used in everything from propellants to refrigeration and air conditioning, foam manufacture, fire extinguishers, and beyond.
These chemicals have the advantage of being very stable (remaining in the atmosphere between 50 and 100 years) and posing no harm to health. However, unlike most pollutants generated by human activity, they are not destroyed in the troposphere and can therefore reach the stratosphere.

Chemists carried out laboratory experiments that demonstrated that chlorine and bromine atoms were able to mix with ozone molecules, thereby destroying them. (They went on to win a Nobel Prize for their discovery.)
But the scientists still had other puzzles to solve. Why was this widespread destruction only occurring over the South Pole? And how come satellites had not raised any warnings?
In the latter case, the computer codes that processed the observations and discarded anomalous data were found to have been systematically rejecting the measured values, as these were much too low compared to the expected concentrations usually measured at the Earth’s poles.
In an effort to shed light on the phenomenon, observation campaigns led to the development of weather balloons and planes for measuring ozone at varying altitudes.
Their observations indicated that the ozone had been completely destroyed across an area of 15 to 20 square kilometres (dubbed the “ozone hole”). But why was this depletion not as significant in higher regions of the atmosphere where the most ozone would be, specifically around 25 km above our heads?
This article is brought to you in partnership with “Your Planet”, an AFP audio podcast. A creation to explore initiatives in favour of ecological transition, all over the planet. Subscribe
1985: an awakening
When the puzzle was solved in 1985, three “ingredients” were revealed to be at play: the dynamics of the stratosphere trapping very cold air masses in the form of vortexes during winter; specific cloud types (i.e., polar stratospheric clouds) caused when temperatures inside these vortexes dropped to approximately -80°C; and greater levels of sunshine in spring, which would trigger a chain of catalytic reactions on the surface of these clouds, involving stable chlorinated and brominated compounds built up over the preceding weeks.
These findings caused a domino effect, culminating in the United Nations signing the Vienna Convention for the Protection of the Ozone Layer that same year. The text recognised the need for greater international cooperation with a view to limiting human-induced damage to this layer. Building on this consensus, the Montreal Protocol came into existence two years later.
While industrialists at the time proposed to use more reactive substitutes that would decompose before reaching the stratosphere, the scientific community undertook to publish scientific reports every four years. These authoritative reports were to collate all available information on the ozone and its evolution.
As things stand, the ozone hole has not yet disappeared, as a considerable amount of CFCs and halons remain in the stratosphere. Nevertheless, concentrations of these are declining rapidly, and scientists today regard the ozone layer as being “in remission”. Indeed, measurements have shown for several years now that the ozone hole is no longer widening, but gradually shrinking.
Current knowledge predicts that it will be back in balance sometime between 2060 and 2070, once all the offending substances have disappeared from the stratosphere.
Potential delays
There are, however, several reasons for this rebalance to come into effect at a later date than indicated in current forecasts. The first is that all countries must stick to their commitments. In 2018, based on data from monitoring stations dotted across the planet, researchers detected that concentrations of CFC-11 were not falling as quickly as they should have been. Using back trajectory analysis, they ascertained that the relevant emissions were coming from provinces in eastern China.
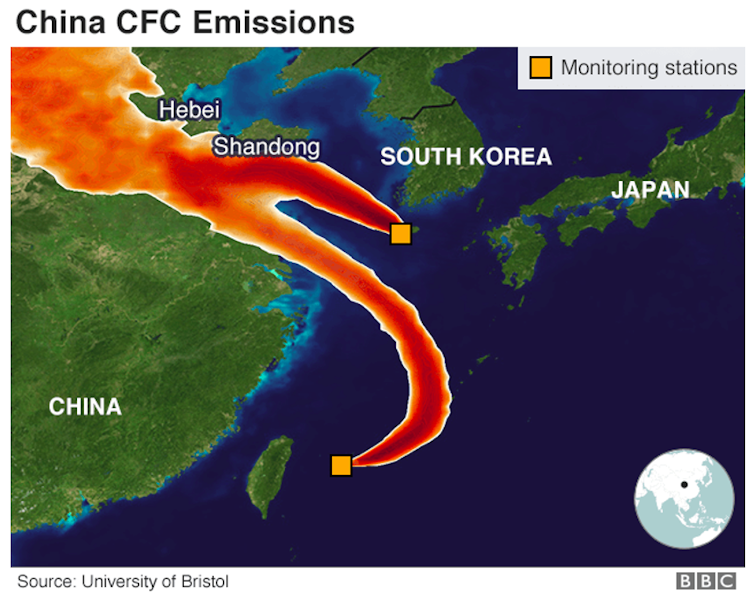
Another cause, which partly explains why the process has already fallen slightly behind initial estimates, relates to the warming of the lower layers of the atmosphere. In essence, when the bottom of the atmosphere absorbs more energy, the stratosphere cools down due to a compensation effect. The colder it becomes, the faster the polar stratospheric clouds form, which in turn destroys more ozone.
The third potential reason is linked to climate engineering, specifically the technique of intentionally sending suspended particles into the stratosphere to mimic a volcanic eruption and deflect some of the Sun’s rays. An experiment was carried out to simulate the injection of particles over Antarctica. Its results found that although the global temperature would fall by 0.5°C, the ozone hole would revert to high levels of damage similar to those observed in the 1990s.
The way forward?
Ozone layer restoration could serve as a template for mitigating climate disruption. It is well established that human-induced greenhouse gas emissions trap infrared radiation and have already caused temperatures to rise.
IPCC reports have been compiling the most recent scientific knowledge for decision-makers every five to six years. Ambitious international agreements such as the 2015 Paris Agreement have been put in place to keep the global temperature rise “well below 2°C”.
But the big difference here is scale. It is not enough to persuade a handful of industrialists to find the right chemical substitutes; what we need is a total sea change in the way our fossil fuel-dependent societies operate.
This article is part of a project between The Conversation France and AFP Audio, supported financially by the European Journalism Centre, as part of the Bill and Melinda Gates Foundation “Solutions Journalism Accelerator” “Solutions Journalism Accelerator” initiative. AFP and The Conversation France have maintained their editorial independence at every stage of the project.
Cathy Clerbaux, Directrice de recherche au CNRS (LATMOS/IPSL), professeure invitée Université libre de Bruxelles, Sorbonne Université
This article is republished from The Conversation under a Creative Commons license. Read the original article.











