At a Glance
- A physicist has settled a century-old debate by proving the Nernst heat theorem stems from the second law of thermodynamics, not a separate third law as believed.
- This finding directly refutes an idea popularized by Albert Einstein, who had detached the theorem from the second law and established it as an independent principle.
- The new proof’s key insight is introducing a virtual engine, a formal thermodynamic concept overlooked in the historic scientific argument.
- With this proof, the unattainability of absolute zero is also shown to be a direct consequence of the second law, unifying these concepts under one principle.
- This breakthrough significantly narrows the scope of the third law of thermodynamics, a change that could lead to significant revisions in how the subject is taught.
A physicist has resolved a 120-year-old debate in thermodynamics, demonstrating that a long-standing principle thought to be a separate law of nature is a direct consequence of the well-established second law. In a paper published in The European Physical Journal Plus, University of Seville professor José Martín-Olalla proves the Nernst heat theorem using only thermodynamic arguments. This finding restructures our understanding of physics at temperatures near absolute zero, the coldest possible limit in the universe at minus 273.15 degrees Celsius. The theorem, first proposed by Walther Nernst in 1905, observes that entropy exchanges — a measure of how energy and disorder are distributed in a system — approach zero as the temperature nears absolute zero.

The historical puzzle began in the early 20th century, pitting Nernst against Albert Einstein. The second law of thermodynamics states that the universe’s total entropy, or disorder, always increases. Nernst argued that his theorem was connected to this law, reasoning that if absolute zero were achievable, one could build a perfectly efficient engine that would violate the second law. Einstein countered that such a machine was impractical and could not be built, leading him to conclude that the Nernst theorem must be a separate third law of thermodynamics. This view has been the standard in physics for more than a century.
Martín-Olalla’s proof resolves this debate by introducing a crucial nuance that both Nernst and Einstein overlooked: the role of a virtual engine. His demonstration relies on a theoretical tool known as a Carnot thermometer, which is formalized by an idealized engine. Crucially, this engine is virtual — it is a thought experiment that does not consume heat or produce work and, therefore, does not violate the second law. By using this formal concept of a temperature scale tied to the second law, Martín-Olalla shows that the Nernst theorem and the unattainability of absolute zero are both necessary outcomes of the second law itself, without needing to invoke a third.
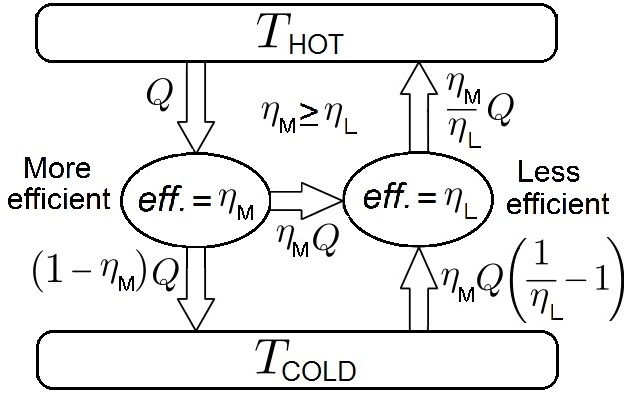
This work proposes a significant revision to physics textbooks, extending the second law’s reach while narrowing the third’s scope. With this proof, the third postulate of thermodynamics is refined to a more specific rule: that the entropy of a chemically uniform body cannot be negative. The other significant observation at low temperatures that the ability of a material to absorb heat also vanishes remains an important appendix but not a fundamental principle. “The students in the thermodynamics course I teach were the first to learn about this demonstration,” Martín-Olalla said. “I hope that with this publication the demonstration will become better known, but I know that the academic world has a great deal of inertia.”
References
- Martín-Olalla, J.-M. (2025). Proof of the Nernst theorem. The European Physical Journal Plus, 140(6), 528. https://doi.org/10.1140/epjp/s13360-025-06503-w
- University of Seville. (2025, June 17). Thermodynamics revisited: Study solves 120-year-old problem and corrects one of Einstein’s ideas. Phys.Org; University of Seville. https://phys.org/news/2025-06-thermodynamics-revisited-year-problem-einstein.html











