
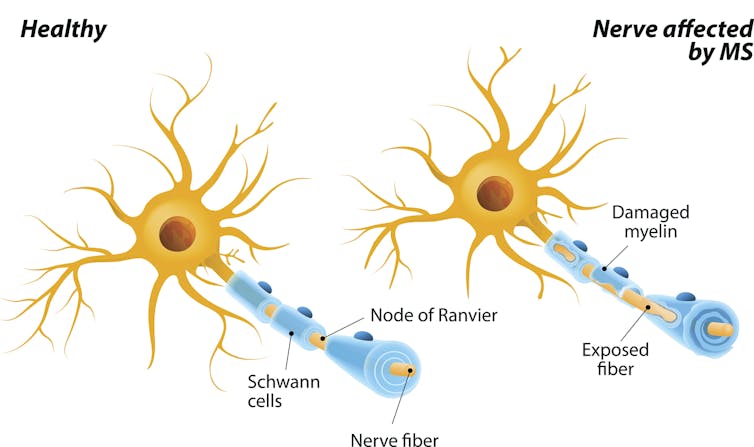
Multiple sclerosis is characterized by an immune system gone haywire. A patient’s immune system starts treating the protective coating of the nerves – called myelin – as dangerous. The subsequent nerve damage can cause a variety of symptoms, including muscle weakness, pain and vision loss. MS currently has no cure, and doctors still don’t completely understand what causes it.
While there is a genetic component to MS, environmental factors also play a big role in determining whether someone will develop the disease. Recent evidence suggests that what’s in your digestive tract may also be a meaningful contributor to disease risk.
My colleagues and I at the University of Virginia are working to understand the two-way communication between the human body and the bacteria that live in its digestive system. In our recently published research, we found that bile acid in the intestines could be harnessed to protect people at high risk of MS from developing the disease, offering a new avenue for drug development.
The gut microbiome and autoimmunity
Trillions of bacteria live in the human gut. They help the body with everything from digesting food to preventing the overgrowth of infectious and dangerous bacteria. They also “educate” the immune system to recognize what is dangerous and what is not. If this process is disturbed, the immune system may become overactive and start to treat natural parts of the body as dangerous. This is called autoimmunity.
Scientists believe that one way bacteria and the immune system communicate with each other is through the aryl hydrocarbon receptor, or AHR, which resides in most cells of the body. This protein acts like an emergency call center – when it encounters certain chemicals, it will identify the appropriate response and send a signal to the cell recommending what it should do.
While researchers have shown that signals from AHR influence multiple sclerosis development, how it does so is unclear. To better understand what AHR is doing specifically in the guts of patients with MS, we genetically engineered mice that are missing AHR in some of their immune cells. By silencing AHR’s activity, we could understand what role it may be playing in autoimmunity.
We expected to learn more from this experiment about the molecular communication of immune cells. Instead we found something surprising: The gut environment in these mice had changed. Specifically, the chemical composition of their guts had been altered, indicating that the metabolism of gut bacteria had shifted. This meant that AHR is not only sensing what’s going on in the gut, but the receptor is also actively shaping its environment.
More importantly, we found that mice without AHR were able to recover from MS. In our mouse model of MS, we induced autoimmunity by immunizing mice against myelin, the protective layer surrounding neurons. This meant that the immune system of the mice was primed to attack myelin, leading to the poor muscle control and paralysis seen in MS. We wanted to test whether the gut microbiome played a role in why mice without AHR were able to recover. When we transplanted the gut bacteria from the digestive tracts of mice without AHR into mice with AHR, we found that the mice with AHR were also able to recover from paralysis. This meant that the gut microbiome was driving recovery from MS.
We also found that the guts of mice without AHR had high levels of bile acids – chemicals produced in the liver and secreted into the intestines that help with digestion. Bile acids are often broken down by the resident bacteria in the gut.
One bile acid in particular, called taurocholic acid, was especially concentrated in mice without AHR. To test whether taurocholic acid offered protection against MS, we fed this chemical to mice with AHR as they started to develop autoimmunity to myelin. While control mice that were fed saline became paralyzed from the waist down, the mice that were fed taurocholic acid just got a little wobbly before they recovered.
With further investigation, we discovered that these mice were able to recover their motor control because their immune cells were not as strong. Exposing their immune cells to bile acids shortened the life span of the cells, thus preventing them from causing as much damage to myelin and motor neurons.
While we still do not understand why bile acids weaken immune cells, we believe it may be a key step to understanding how to interrupt autoimmunity in MS and other autoimmune disorders.
Better treatments for multiple sclerosis
Current available therapies for autoimmune disorders like MS are immunosuppressant drugs that quiet the immune response. While these drugs can reduce relapse and delay disease progression, they can also put patients at high risk of infection and difficult side effects. With the COVID-19 pandemic, the danger of having a weakened immune system has become even more apparent.
Finding other avenues to quiet an overactive immune system, such as through bile acids, could help researchers create safer drugs that could help prevent or treat disease. The body produces eight different bile acids that each have different chemical properties. Our team is working to identify whether taurocholic acid is truly the best option for treating MS or if another bile acid – or a combination of several – would be more effective.
Bile acids are far from ready to be used as treatment in people. But we believe that the key to preventing multiple sclerosis may be inside us already.
Andrea Merchak, Ph.D. Candidate in Neuroscience, University of Virginia
This article is republished from The Conversation under a Creative Commons license. Read the original article.











