Heart disease is the leading cause of death worldwide. The World Health Organization estimates that 17.9 million people lose their lives to it each year, accounting for 32% of global deaths.
Doris Taylor is a scientist working in regenerative medicine and tissue engineering. Her work has focused on creating personalized functioning human hearts in a lab that could rule out the need for donors. Taylor has dubbed these hearts “ghost hearts.”
In March, Taylor spoke at the 2023 Imagine Solutions Conference in Naples, Florida, about the ghost heart and her journey to creating it. Below are edited answers to questions from The Conversation. Taylor is also featured on Guy Kawasaki’s Remarkable People podcast.
What are the biggest challenges facing organ donations today?
Currently, patients in need of a heart transplant need to join a waitlist, and hearts become available when someone else has died. Because there are not enough hearts to go around, only the very sick are put on the waitlist. The U.S. transplants about 11 hearts a day, and on a given day there are more than 3,000 people waiting for a heart.
Even when organs are successfully transplanted, it isn’t a Hollywood fairy-tale ending. A person receiving an organ transplant essentially trades one disease for other medical complications and diseases. Toxic drugs necessary to prevent rejection can cause high blood pressure diabetes, cancer and kidney failure. These are serious medical issues that also affect people emotionally, financially and physically.
About 18% of people die in the first year after a transplant.
What is the so-called “ghost heart”? How does it work?
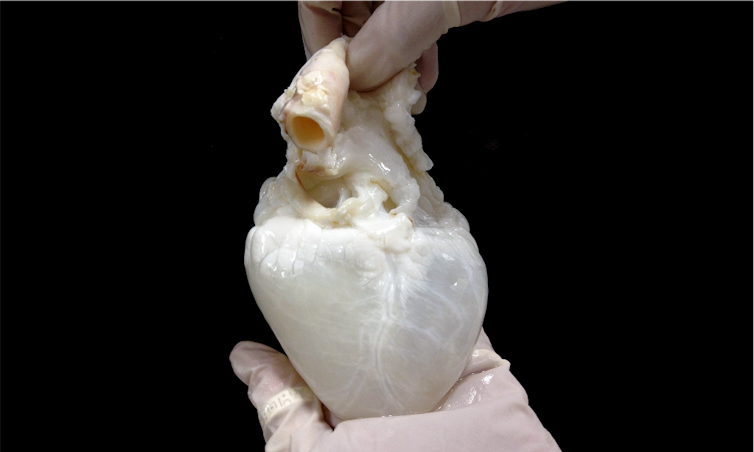
The ghost heart is a heart whose cells have been removed. All that remains is the heart framework, or scaffolding. It’s called a ghost heart because removing the cells causes the heart to turn from red to white. A human heart wouldn’t work as a scaffold because so few are available to work with.
So my team and I went with the next best thing: a pig heart. Pig hearts are similar to human hearts in terms of their size and structure. Both have four chambers – two atria and two ventricles – responsible for pumping blood. And structures from pig hearts such as valves have been used in humans safely.
To remove the cells, the pig heart is gently washed through its blood vessels with a mild detergent to remove the cells. This process is called perfusion decellurization. The cell-free heart can then be seeded with new cells – in this case, a patient’s cells – thus forming a personalized heart.
What role do stem cells play in creating a heart?
If you lined up the cells needed for an average-size 350-gram human heart, they would stretch for 41,000 miles. Stacked on top of one another, they would amount to 2 billion lines of cells, or enough to fill seven movie screens. But heart cells don’t divide. If they did, hearts could likely repair themselves.
Stem cells, on the other hand, do divide. They can also form into specialized cells – in this case, heart cells. Nobel Prize laureate Dr. Shinya Yamanaka discovered a method to make stem cells out of blood or skin cells from an adult. My team and I employed this method to obtain stem cells, then grew those cells into billions. After that, the team used chemicals to “differentiate” them into heart cells. We employed this method to obtain billions and billions of heart cells.
The first time I saw heart cells beating in a dish it was life-changing. But while the cells are alive and beat, they are not a heart. To be a heart, these cells need to be placed into a form that lets them become a unified organ, to mature and to be able to pump blood. In a human body, this happens during development; we had to reproduce that capacity in the lab.
In 2022, a pig heart that had been genetically engineered to reduce rejection and improve acceptance was transplanted into a human. Why is it better to build a heart from scratch using pig scaffolding instead?
Let me be clear: Any heart is better than no heart. And xenotransplantation – the process by which nonhuman animal organs are transplanted into humans – opened doors for all scientists in this field.
The patient received a pig heart that had been gene-edited. Human genes were added, and some pig genes were removed, but the heart still essentially comprised pig cells within a pig scaffold. As a result, the individual had to take anti-rejection drugs that suppressed the immune system. And, unbeknownst to doctors, the heart was carrying a pig virus that ultimately killed the patient two months following the transplant.
I believe these sorts of problems are avoided with the ghost heart. My team removes the pig cellular material from the scaffold, leaving only the protein structure and blood vessel channels behind. The proteins are so similar to human scaffold proteins they that don’t appear to cause rejection.
What are the biggest challenges facing the ghost heart effort?
My team and I have encountered two major hurdles. The first is the time and cost it takes to grow the cells.
The second is enabling the heart to mature once the cells are delivered into it – all while maintaining sterility in the absence of any antibiotics. My lab and our partners have had to essentially recreate the heart outside of the body and build the equivalent of an artificial human body that provides food, temperature control, oxygen and other nutrients as well as a blood pressure and artificial blood flow – we call it a biocradle – in which to place the heart. We have to train the immature heart cells to work together even as we are coaxing them to grow strong enough to pump blood; figure out how to feed them and get oxygen to them without lungs; and keep them sterile without an immune system. It’s a huge endeavor.
I liken it to a symphony in which each section has to come in at just the right time to create a beautiful, complex song – but if one piece isn’t ready, the whole thing falls apart. My job is to be the conductor.
Where do you see the future of organ donations in 30 years?
Today, organ donation is lagging behind need. Scientists are aiming to change that by increasing the number of donors, making more organs available by rejuvenating those that can’t be used and by building new technology – as my team and I are doing with the ghost heart. But it’s more than supply and demand. Access is not equal. In fact, organ transplant is a huge health inequity issue. Today, the organ transplant system fails people of color. For example, African Americans have a higher rate of heart failure but are less likely to receive hearts.
As science evolves, scientists have the opportunity to make organs accessible and to deliver organs that don’t require expensive toxic drugs. I look forward to that day and work daily to help create it.
Most people know months to years in advance that they need a transplant. In general, the current wait for a heart is about a year for white Americans but longer for African Americans, while the data for Latinos and Asians is less clear. For other organs, the wait can be three to five years. Not only is that a long time – it is an inequitable time that needs to change.
Building a heart is a 24/7, 365-day-a-year effort. A dedicated team and I, along with supporters, have the opportunity to build hearts earlier and change heart transplants from emergency procedures to planned hospital surgeries – and to do so equitably.
Doris Taylor, Regenerative Medicine Lecturer, University of New Hampshire
This article is republished from The Conversation under a Creative Commons license. Read the original article.










