
If you hadn’t heard about superconductors before 2023, odds are you know what they are now. Researchers raised eyebrows early in the year with claims of operational room-temperature superconductors, though none has been substantiated, and one paper from researchers at the University of Rochester was retracted by the journal Nature at the authors’ request in November.
But the hunt for a superconductor – that is, a material that can conduct electricity without resistance – that can operate at room temperature is nothing new.
Right now, superconductors can operate only at very cold temperatures. So, finding one that could work at room temperature without needing to be kept in a cold chamber could revolutionize everything from power grids and medical equipment to quantum computing. But physicists first have to figure out how to make them work.
A Dutch physicist discovered the phenomenon of superconductivity in the early 20th century, and since then, labs around the world have tested materials that can reach a superconductive state at warmer and warmer temperatures.
So, how do these materials manage to conduct electricity without resistance, and what sorts of technological possibilities lie on the horizon, with superconductor research improving every year? Here are three stories from The Conversation’s archive that explore the history, science and future of this incredible physical phenomenon.
1. Physics behind the phenomenon
How is it even possible to generate a current with zero electrical resistance, the basis for superconductivity? In order to do so, you must keep your conducting metal cold. Really cold. Like, hundreds of degrees below zero.
“At normal temperatures, electrons move in somewhat erratic paths. They can generally succeed in moving through a wire freely, but every once in a while they collide with the nuclei of the material,” wrote Mishkat Bhattacharya, a physicist at Rochester Institute of Technology. “These collisions are what obstruct the flow of electrons, cause resistance and heat up the material.”
Normally, the nuclei of all atoms vibrate constantly, and they can bump into each other. In superconducting materials, the electrons in the current pass from atom to atom while vibrating at the same frequency as the nuclei of the atoms in the superconducting metal. This means that instead of colliding and generating heat, they’re moving in a smooth and coordinated way. And it’s the cold temperatures that allow for this coordinated movement.
2. A century of superconductivity
Mercury was the first material discovered as a superconducter, by Heike Kamerlingh Onnes in 1911. His team had to cool liquid helium to -454 degrees Fahrenheit (-270 degrees Celsius) to observe the effect. They used wires made of mercury to send a current through the material, and then measured the effect of electrical resistance as “near enough null.”
Onnes and his team repeated the experiment several times to make sure the effect they’d observed was, in fact, superconductivity, and they also troubleshot all other possible explanations for the effect – electrical faults, open currents and so on. But they kept finding the same result, and after three years of testing, Onnes was able to demonstrate currents with truly zero resistance.
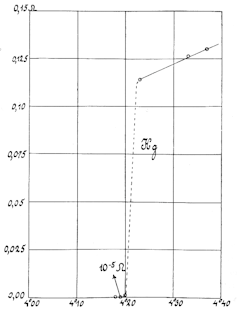
“Superconductivity has always been tricky to prove because some metals can masquerade as superconductors,” wrote David D. Nolte, an author of history of science books and a physicist at Purdue. “The lessons learned by Onnes a century ago – that these discoveries require time, patience and, most importantly, proof of currents that never stop – are still relevant today.”
3. A superconductive future
One of the most important applications of a future room-temperature superconductor would be decreasing the heat wasted from electronics. Not only could electronics like cellphones and computers run much more quickly and efficiently, but on a larger scale, electric grids, power lines and data centers could decrease their wasted heat. This could be a huge win for the environment.
“If we succeed in making a room-temperature superconductor, then we can address the billions of dollars that it costs in wasted heat to transmit energy from power plants to cities,” wrote Pegor Aynajian, a physicist at Binghamton University, State University of New York. “Solar energy harvested in the vast empty deserts around the world could be stored and transmitted without any loss of energy, which could power cities and dramatically reduce greenhouse gas emissions.”
A type of superconductor made from a ceramiclike material discovered by scientists at IBM in Switzerland could be one path to a room-temperature superconductor. Already, this class of materials has been shown to work at higher – though still frigid – temperatures, closer to -300 F (-184 C) than conventional superconductors like Onnes’ original mercury wires.
But while a room-temperature superconductor could revolutionize electronics and energy transmission, the right material still remains elusive. As Aynajian puts it, a room-temperature superconductor is quite literally “the next million-dollar question.”
This story is a roundup of articles from The Conversation’s archives.
Mary Magnuson, Assistant Science Editor, The Conversation
This article is republished from The Conversation under a Creative Commons license. Read the original article.











